AEROSOL THERAPY FOR ASTHMA
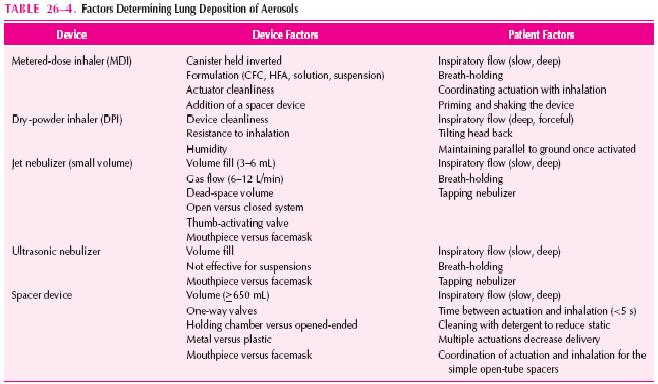
Aerosol delivery of drugs for asthma has the advantage of being site-specific and thus enhancing the therapeutic ratio. Inhalation of short-acting β2-agonists provides more rapid bronchodilation than either parenteral or oral administration, as well as the greatest degree of protection against EIB and other challenges. Inhaled corticosteroids produce a greater reduction of BHR than corticosteroids administered systemically. Specific agents (e.g., cromolyn, nedocromil, formoterol, salmeterol, and ipratropium) are only effective by inhalation. Given the international ban on the production and use of chlorofluorocarbons (CFCs), the manufacturers of CFC-propelled metered-dose inhalers (MDIs) are developing new devices for delivering topically active medication. Therefore, an understanding of aerosol drug delivery is essential to optimal asthma therapy. Table 26-4 lists the factors determining lung deposition of therapeutic aerosols.
DEVICE DETERMINANTS OF DELIVERY
Devices used to generate therapeutic aerosols include jet nebulizers, ultrasonic nebulizers, MDIs, and dry-powder inhalers (DPIs).
DEVICE DETERMINANTS OF DELIVERY
Devices used to generate therapeutic aerosols include jet nebulizers, ultrasonic nebulizers, MDIs, and dry-powder inhalers (DPIs).
The single most important device factor determining the site of aerosol deposition is particle size. Devices for delivering therapeutic aerosols generate particles with aerodynamic diameters from 0.5 to 35 microns. Particles larger than 10 microns deposit in the oropharynx, particles between 5 and 10 microns deposit in the trachea and large bronchi, particles 1 to 5 microns in size reach the lower
airways, and particles smaller than 0.5 microns act as a gas and are exhaled. In asthma, the airways, not the alveoli, are the target for delivery. Respirable particles are deposited in the airways by three mechanisms:
(1) inertial impaction,
(2) gravitational sedimentation, and
(3) Brownian diffusion.
The first two mechanisms are the most important for therapeutic aerosols and probably are the only factors that can be manipulated by patients. Each delivery device within a classification generates specific aerosol characteristics, so extrapolation of delivery data from one device cannot be done for the other devices in the class. For instance, MDIs can deliver 5% to 50% of the actuated dose; DPIs, 10% to 30% of the labeled dose; and nebulizers, 2% to 15% of the starting dose. MDIs and DPIs are portable and convenient, unlike nebulizers. MDIs consist of a pressurized canister with a metering valve; the canister contains active drug, low-vapor-pressure propellants such as chlorofluorocarbon (CFC) or hydrofluoroalkane (HFA), cosolvents, and/or surfactants. With any change in these components, the Food and Drug Administration (FDA) considers it to be a new drug that requires stability, safety, and efficacy studies prior to approval. The drug is either in solution or a suspended micronized powder. In order to disperse the suspension for accurate delivery, the canister must
be shaken. The metering chamber measures a liquid volume, and therefore, the device must be held with the valve stem downward so that the chamber is covered with liquid (Fig. 26-4). When the
canister is actuated, the device releases the propellant and drug in a forceful spray whose particles are large (mass median aerodynamic diameter [MMAD] = 45 microns) (see Fig. 26–4). As evaporation
occurs, the particle size is reduced to a final MMAD of 0.5 to 5.5 microns depending on the MDI. The aerosol cloud of a CFCpropelled MDI extends at least 10 in beyond the MDI at the lowest
MMAD, and that of an HFA-propelled MDI extends about 6 in. The breath-actuated MDI Autohaler, is cocked with a lever to “load” the dose of medication, a baffle is opened by inspiratory pressure,
and the dose is expelled from the canister metering chamber. While the need for hand-lung coordination for proper actuation is reduced significantly with breath-actuated MDIs, these devices do not allow the use of a spacer device.
airways, and particles smaller than 0.5 microns act as a gas and are exhaled. In asthma, the airways, not the alveoli, are the target for delivery. Respirable particles are deposited in the airways by three mechanisms:
(1) inertial impaction,
(2) gravitational sedimentation, and
(3) Brownian diffusion.
The first two mechanisms are the most important for therapeutic aerosols and probably are the only factors that can be manipulated by patients. Each delivery device within a classification generates specific aerosol characteristics, so extrapolation of delivery data from one device cannot be done for the other devices in the class. For instance, MDIs can deliver 5% to 50% of the actuated dose; DPIs, 10% to 30% of the labeled dose; and nebulizers, 2% to 15% of the starting dose. MDIs and DPIs are portable and convenient, unlike nebulizers. MDIs consist of a pressurized canister with a metering valve; the canister contains active drug, low-vapor-pressure propellants such as chlorofluorocarbon (CFC) or hydrofluoroalkane (HFA), cosolvents, and/or surfactants. With any change in these components, the Food and Drug Administration (FDA) considers it to be a new drug that requires stability, safety, and efficacy studies prior to approval. The drug is either in solution or a suspended micronized powder. In order to disperse the suspension for accurate delivery, the canister must
be shaken. The metering chamber measures a liquid volume, and therefore, the device must be held with the valve stem downward so that the chamber is covered with liquid (Fig. 26-4). When the
canister is actuated, the device releases the propellant and drug in a forceful spray whose particles are large (mass median aerodynamic diameter [MMAD] = 45 microns) (see Fig. 26–4). As evaporation
occurs, the particle size is reduced to a final MMAD of 0.5 to 5.5 microns depending on the MDI. The aerosol cloud of a CFCpropelled MDI extends at least 10 in beyond the MDI at the lowest
MMAD, and that of an HFA-propelled MDI extends about 6 in. The breath-actuated MDI Autohaler, is cocked with a lever to “load” the dose of medication, a baffle is opened by inspiratory pressure,
and the dose is expelled from the canister metering chamber. While the need for hand-lung coordination for proper actuation is reduced significantly with breath-actuated MDIs, these devices do not allow the use of a spacer device.
Spacer devices are used frequently with an MDI to decrease oropharyngeal deposition and enhance lung delivery. However, not all spacer devices produce similar effects. The design of spacers varies from simple open-ended tubes that separate the MDI from the mouth to holding chambers with one-way valves that open during inhalation (the preferred system). The purpose of a spacer is to allow evaporation of the propellant prior to inhalation. Use of a spacer allows inhalation after actuation of the device, obviating the need for good hand-lung coordination, and permits a greater number of drug particles to achieve a respirable droplet size. Additionally, the largeparticles that normally would deposit in the oropharynx “rain out” in the spacer. All the available spacers significantly reduce oropharyngeal deposition from MDIs, with the holding-chamber devices being
superior to the open-ended tubes. This reduction in oropharyngeal deposition is an important factor in reducing local adverse effects (i.e.,hoarseness and thrush) from inhaled corticosteroids. The change in lung delivery depends on both the MDI and the drug, where one spacer device may enhance delivery with one MDI preparation and decrease delivery with others. Finally, over time, holding chambers can build up static electricity that attracts small particles to the sides of the chamber, significantly reducing aerosol availability. It is recommended that spacers be washed weekly with household detergent, with a single rinse, and allowed to drip dry. Dry micronized powders can be inhaled directly into the lung. Four DPIs for asthma are available for use in the United States (i.e., Diskus, Rotahaler, Turbuhaler, and Aerolizer), with others under development. Each has unique characteristics with advantages and disadvantages (Table 26–5). The primary advantage of DPIs is that they are breath-actuated and require minimal hand-lung coordination. Some DPIs are more flow-dependent than others. Thus, similar to MDIs and spacers, delivery data from one DPI cannot
be extrapolated to another. Nebulizers come in two basic types, the jet nebulizer and the ultrasonic nebulizer. Jet nebulizers produce an aerosol from a liquid solution or suspension placed in a cup. A tube connected to a stream of compressed air or oxygen flows up through the bottom and draws the liquid up an adjacent open-ended tube. The air and liquid strike a baffle, creating a droplet cloud that is then inhaled. Ultrasonic nebulizers produce an aerosol by vibrating liquid lying above a transducer
at speeds of about 1 mHz. Both produce similar degrees of lung deposition, with the exception that ultrasonic nebulizers are ineffective for nebulizing micronized suspensions. The aerosol output and
lung delivery vary significantly among the commercially available jet nebulizers even when operated in the same manner. Increasing fill volume will increase the total amount of drug delivered; however, it
also will take longer for the patient to nebulize the dose. TheMMAD of the droplets is related directly to the gas flow, with flows of 5 to 12L/ min providing an aerosol cloud with an MMAD of 4 to 8 microns for most jet nebulizers.
superior to the open-ended tubes. This reduction in oropharyngeal deposition is an important factor in reducing local adverse effects (i.e.,hoarseness and thrush) from inhaled corticosteroids. The change in lung delivery depends on both the MDI and the drug, where one spacer device may enhance delivery with one MDI preparation and decrease delivery with others. Finally, over time, holding chambers can build up static electricity that attracts small particles to the sides of the chamber, significantly reducing aerosol availability. It is recommended that spacers be washed weekly with household detergent, with a single rinse, and allowed to drip dry. Dry micronized powders can be inhaled directly into the lung. Four DPIs for asthma are available for use in the United States (i.e., Diskus, Rotahaler, Turbuhaler, and Aerolizer), with others under development. Each has unique characteristics with advantages and disadvantages (Table 26–5). The primary advantage of DPIs is that they are breath-actuated and require minimal hand-lung coordination. Some DPIs are more flow-dependent than others. Thus, similar to MDIs and spacers, delivery data from one DPI cannot
be extrapolated to another. Nebulizers come in two basic types, the jet nebulizer and the ultrasonic nebulizer. Jet nebulizers produce an aerosol from a liquid solution or suspension placed in a cup. A tube connected to a stream of compressed air or oxygen flows up through the bottom and draws the liquid up an adjacent open-ended tube. The air and liquid strike a baffle, creating a droplet cloud that is then inhaled. Ultrasonic nebulizers produce an aerosol by vibrating liquid lying above a transducer
at speeds of about 1 mHz. Both produce similar degrees of lung deposition, with the exception that ultrasonic nebulizers are ineffective for nebulizing micronized suspensions. The aerosol output and
lung delivery vary significantly among the commercially available jet nebulizers even when operated in the same manner. Increasing fill volume will increase the total amount of drug delivered; however, it
also will take longer for the patient to nebulize the dose. TheMMAD of the droplets is related directly to the gas flow, with flows of 5 to 12L/ min providing an aerosol cloud with an MMAD of 4 to 8 microns for most jet nebulizers.
PATIENT DETERMINANTS OF DELIVERY (See Table 26–4)
The most important patient factor determining aerosol deposition is inspiratory flow. High inspiratory flows increase the degree of deposition owing to impaction of particles of any size, thereby increasing deposition centrally (i.e., throat and large airways) and decreasing peripheral deposition. Holding chambers enhance the clinical efficacy in patients with poor hand-lung coordination but may offer no advantage in patients who can use an MDI optimally alone. Optimal inspiratory flow for most MDIs is
slow and deep (approximately 30 L/min). In general, DPIs require
slow and deep (approximately 30 L/min). In general, DPIs require
higher inspiratory flows (≥60 L/min) and a change in inhalation technique (i.e., deep, forceful inspiration) for optimal actuation, which, in turn, increases the amount of drug delivered to the larger central airways. However, this difference in delivery may not produce clinically significant differences. Patients should be cautioned not to exhale into DPIs because this causes loss of dose and moistens the dry powder, causing aggregation into larger particles. Patient factors that cannot be controlled include interpatient variability in airway geometry (particularly the differences between children and adults) and the effects of bronchospasm, edema, and mucus hypersecretion. Mild
obstruction increases aerosol deposition; however, severe obstruction probably leads to increased central deposition from impaction. The absolute delivery to the lung is not as important as consistency of delivery, assuming that a sufficient dose to produce the desired therapeutic
effect is achieved. No single inhalation device is the best for all patients. Table 26–5 lists the differing characteristics of inhalation devices. Appropriate inhalation technique is essential to achieve optimal
drug delivery and therapeutic effect. The components are illustrated in Figure 26–5. Approximately 50% to 80% of a dose from MDIs and DPIs impacts on the oropharynx and is then swallowed;
the rest is either left in the device or exhaled. It is important that actuation occurs during inhalation, although the time during inspiration is unimportant. Although radiolabeled studies indicate improved
delivery by holding the actuator 2 to 3 cm in front of an open mouth to allow more evaporation and less impaction, physiologic studies with bronchodilators have failed to document an advantage for this
method. Many patients do not use their MDIs optimally, and patient instruction with demonstration is the most effective means of improving inhaler technique. Even with instruction, up to 30% of
patients, particularly young children and the elderly, cannot master the use of an MDI. For these patients, attachment of a holding-chamber device to the MDI or use of a breath-actuated MDI can improve effi- cacy significantly. Mouth rinsing following treatment with MDIand DPI-inhaled corticosteroids is important to minimize local effects and oral absorption. Delivery from high-resistance DPIs is more flow-dependent than from low-resistance DPIs. The Turbuhaler device has a greater flow dependency than the Aerolizer and the Diskus. Children 6 to 12 years of age received approximately one-half the dose of radiolabeled budesonide via Turbuhaler than those older than 12 years of age, whereas children younger than 6 years of age received
obstruction increases aerosol deposition; however, severe obstruction probably leads to increased central deposition from impaction. The absolute delivery to the lung is not as important as consistency of delivery, assuming that a sufficient dose to produce the desired therapeutic
effect is achieved. No single inhalation device is the best for all patients. Table 26–5 lists the differing characteristics of inhalation devices. Appropriate inhalation technique is essential to achieve optimal
drug delivery and therapeutic effect. The components are illustrated in Figure 26–5. Approximately 50% to 80% of a dose from MDIs and DPIs impacts on the oropharynx and is then swallowed;
the rest is either left in the device or exhaled. It is important that actuation occurs during inhalation, although the time during inspiration is unimportant. Although radiolabeled studies indicate improved
delivery by holding the actuator 2 to 3 cm in front of an open mouth to allow more evaporation and less impaction, physiologic studies with bronchodilators have failed to document an advantage for this
method. Many patients do not use their MDIs optimally, and patient instruction with demonstration is the most effective means of improving inhaler technique. Even with instruction, up to 30% of
patients, particularly young children and the elderly, cannot master the use of an MDI. For these patients, attachment of a holding-chamber device to the MDI or use of a breath-actuated MDI can improve effi- cacy significantly. Mouth rinsing following treatment with MDIand DPI-inhaled corticosteroids is important to minimize local effects and oral absorption. Delivery from high-resistance DPIs is more flow-dependent than from low-resistance DPIs. The Turbuhaler device has a greater flow dependency than the Aerolizer and the Diskus. Children 6 to 12 years of age received approximately one-half the dose of radiolabeled budesonide via Turbuhaler than those older than 12 years of age, whereas children younger than 6 years of age received
one-quarter of the dose. The Aerolizer and the Diskus deliver about 15% of the dose to the airways, and this appears to be similar at both 30 and 60 L/min inspiratory flows; both have been approved for children aged 5 and 4 years, respectively.



A relevant but strangely ignored or not generally known fact about asthma and breathing troubles is that the change between weak (asthmatic) and strong (healthy) breathing is dependent on abdominal muscle tension. Slackening the muscles here causes abysmally weak and asthmatic breathing. Instead of describing an asthma attack as being like breathing through a straw, attempting to breathe vigorously with relaxed abdominal muscles provides a more genuine illustrative example. Training the muscles, for example by “abdominal hollowing” (see Web articles) produces an antiasthmatic effect. Abdominal muscle tension plays a prominent part in Asian martial arts.
ReplyDeleteI tend to breathe asthmatically after an evening meal or in pollen-laden air.
So it is fair to assume that there is a natural breathing spectrum with an asthmatic tendency at one end and Ku Fu or Karate breathing at the other end. For a few words on the Japanese version of Asian breathing see http://www.lrz.de/~s3e0101/webserver/webdata/OBT.pdf
Breathing powerfully into my lower abdomen with tensed muscles provides an effective cure for me. But then I’ve always been sceptical about medical wisdom on asthma: such a paradoxical and doctor-baffling increase in the last 40 years with modern, merely symptomatic inhalers. Respectfully, Richard Friedel
thank you for the information Mr Richard Friedel ^_^
ReplyDeleteI could not refrain from commenting. Exceptionally well written!
ReplyDeleteMy page; the best way to last longer in bed